
ILOILO – “I want to help so I volunteered.”
So said an Ilonggo nurse based in the United Arab Emirates (UAE) who volunteered to be part of a clinical trial for a potential coronavirus disease 2019 (COVID-19) vaccine.
The 32-year-old Ramon Francisco Sonico is a resident of Santa Barbara town and a graduate of Central Philippine University (2008). He works at a private hospital in the oil-rich Gulf state.
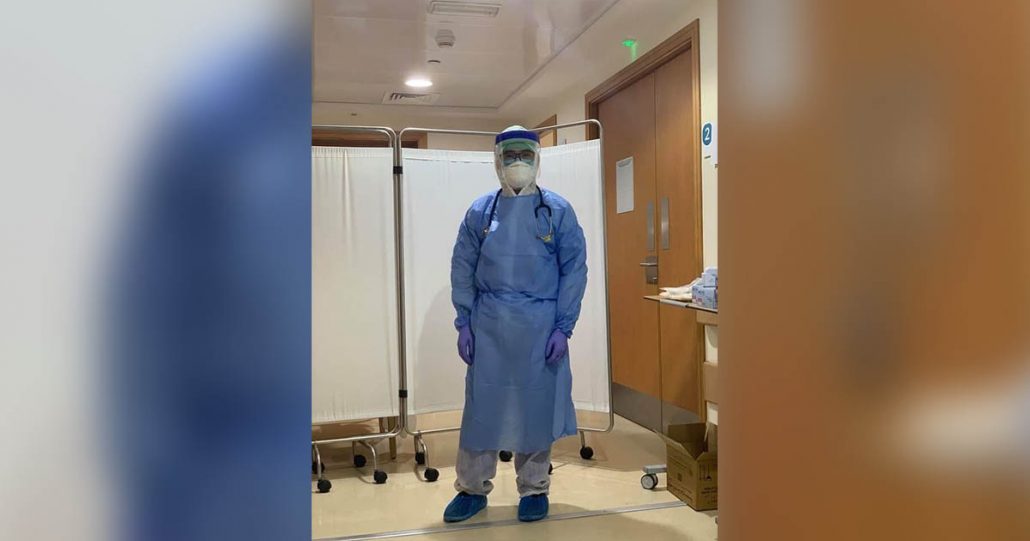
“This is a risk but I want to be part of the solution,” Sonico told Panay News.
Sonico, assigned at the Intensive Care Unit of the hospital with COVID-positive patients on ventilators and on dialysis machines, was inoculated on Aug. 8 with an inactivated SARS-CoV-2 vaccine (vero cell) developed by Sinopharm of China and approved by UAE’s Department of Health for clinical trial.
“I want the pandemic to end so we can go back to our normal lives,” he said.
Prior to the inoculation, Sonico said he went through several processes.
“They took all my personal details, after which I waited for a couple of minutes for the medical checkup. After my vital signs were checked, the doctor asked about my medical history like allergies to food, drugs, hypertension…if I previously had COVID,” according to Sonico.
Afterwards he was brought a room for blood extraction. Sonico said his blood was checked for antibodies, and then he was swabbed.
“I waited again for some 10 to 20 minutes and they injected me the vaccine,” said Sonico.
He was then told to regularly monitor his body temperature and record them.
A doctor is also constantly monitoring his condition, said Sonico.
“So far I feel completely fine. Let’s hope this trial will have a positive result and make the world COVID-free. All for humanity,” according to Sonico.
He would be receiving his second vaccine shot on Aug. 29.
Overall, the monitoring would last for some three to six months after which he would undergo blood test and another swab.
Sonico said he had not contracted COVID-19 since the pandemic started; if he tests positive for it, that means the vaccine he was inoculated with was a failure.
How did he learn about the vaccine trials?
The UAE’s health department advertised it was looking for volunteers, said Sonico.
He registered and was eventually contacted on July 28 that he got qualified.
Sonico said over 45,000 registered to become volunteers but only 15,000 were approved.
“I was initially afraid. In clinical trials, there’s a 50-50 chance of success and failure. But I did a lot of research and asked others who already received the vaccine,” said Sonico.
He informed his family about the clinical trial only after he completed the first inoculation.
“They were all worried. But I pray this will be a success,” said Sonico who has been working in the UAE these past five years./PN





